
DOSING
THE ONLY TRK* INHIBITOR WITH BOTH CAPSULES AND AN ORAL SOLUTION1
AVAILABLE IN CAPSULES AND AN ORAL SOLUTION1
100-mg and 25-mg
capsules1
20-mg/mL oral
solution1
VITRAKVI® can be taken with or without food1

Pre-mixed oral solution is available in strawberry flavor.1,a
*TRK, tropomyosin receptor kinase.
aAvailable in the 2x50-mL solution only.1
VITRAKVI is taken twice daily1
Adult1
100 mg taken orally twice daily until disease progression or until unacceptable toxicity occurs.
Pediatric1
- Recommended dosage in pediatric patients with body surface area of ≥1.0 m2: 100 mg taken orally twice daily until disease progression or until unacceptable toxicity
- Recommended dosage in pediatric patients with body surface area <1.0 m2: 100 mg/m2 taken orally twice daily until disease progression or until unacceptable toxicity
VITRAKVI CAPSULES OR ORAL SOLUTION MAY BE USED INTERCHANGEABLY.1
RECOMMENDED DOSE REDUCTIONS FOR ADVERSE REACTIONS1
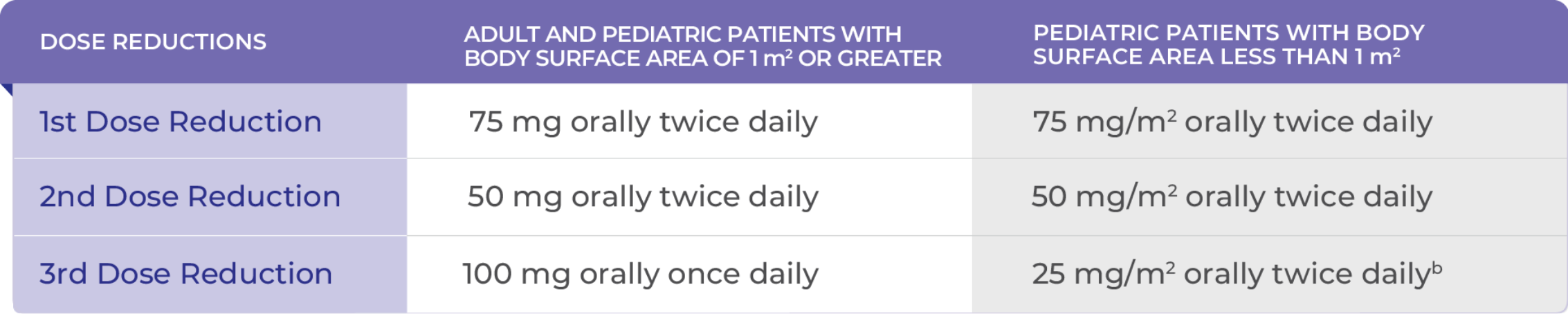
bPediatric patients on 25 mg/m2 orally twice daily should remain on this dose even if body surface area becomes >1.0 m2 during the treatment. Maximum dose should be 25 mg/m2 orally twice daily at the third dose modification.1
For Grade 2 and higher liver function test abnormalities, refer to the Recommended Dose Modifications for Hepatotoxicity table below.1
- For all other Grade 3 or 4 adverse reactions1:
- Withhold VITRAKVI until adverse reaction resolves or improves to baseline or Grade 1. Resume at the next dose modification if resolution occurs within 4 weeks
- Permanently discontinue VITRAKVI if an adverse reaction does not resolve within 4 weeks
- Permanently discontinue VITRAKVI in patients who are unable to tolerate VITRAKVI after 3 dose modifications1
For CTCAE Grade 2 ALT and/or AST elevation, monitor liver function frequently as clinically indicated to establish whether a dose interruption or reduction is required.1
Recommended Dose Modifications for Hepatotoxicity1
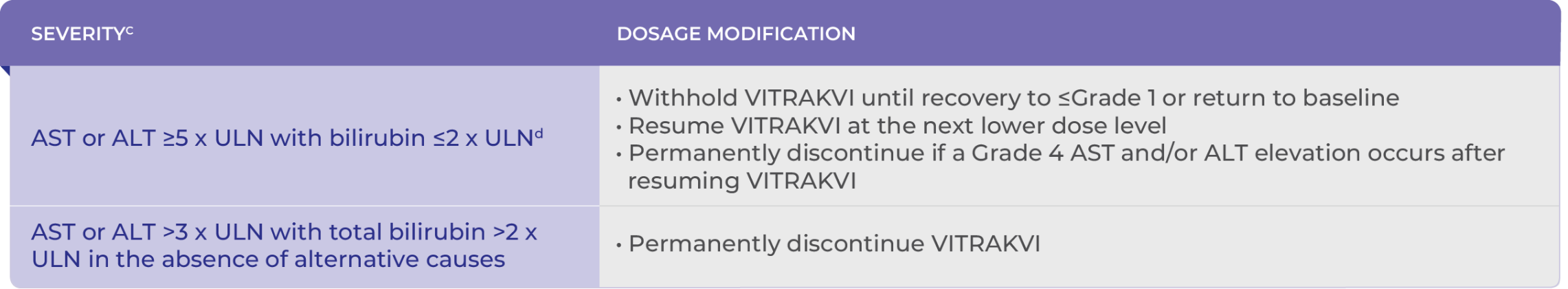
cALP, alkaline phosphatase; ALT, alanine transaminase; ULN, upper limit of normal.
dGrading defined by NCI-CTCAE version 4.03.1
Dosage modifications for coadministration with strong or moderate CYP3A4 inhibitors1
- Avoid coadministration of strong CYP3A4 inhibitors with VITRAKVI. If coadministration of a strong CYP3A4 inhibitor cannot be avoided, reduce the VITRAKVI dose by 50%. After the inhibitor has been discontinued for 3 to 5 elimination half-lives, resume the VITRAKVI dose taken prior to initiating the CYP3A4 inhibitor
- For coadministration with moderate CYP3A4 inhibitors, monitor for adverse reactions more frequently and reduce the dosage based on severity
Dosage modifications for coadministration with strong or moderate CYP3A4 inducers1
- Avoid coadministration of strong CYP3A4 inducers with VITRAKVI. If coadministration of a strong CYP3A4 inducer cannot be avoided, double the VITRAKVI dose. After the inducer has been discontinued for 3 to 5 elimination half-lives, resume the VITRAKVI dose that was used prior to initiating the CYP3A4 inducer. For coadministration with moderate CYP3A4 inducers, modify dose as recommended
Dosage modifications for patients with hepatic impairment1
- Reduce the starting dose of VITRAKVI by 50% in patients with moderate (Child-Pugh B) to severe (Child-Pugh C) hepatic impairment
Reference
- VITRAKVI [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals, Inc.; April 2025. Return to content



