SAFETY
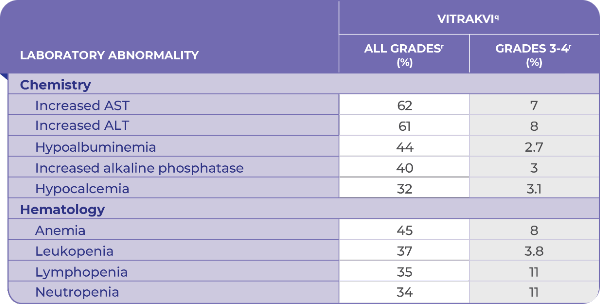
ADVERSE REACTIONS OCCURRING IN ≥10% OF PATIENTS TREATED WITH VITRAKVI®1

- 12% of patients permanently discontinued treatment due to adverse reactions1
- 20% of patients were 65 years and older; no overall differences in safety or effectiveness were observed between patients 65 years and older and younger adult patients1
NO CARDIAC MONITORING REQUIRED WITH VITRAKVI1
Obtain liver function tests (ALT,p AST,p ALP,p and bilirubin) before initiation of treatment and every 2 weeks during the first 2 months of treatment, then monthly thereafter or more frequently following the occurrence of Grade 2 or greater AST or ALT elevation.1
Clinically relevant adverse reactions occurring in ≤10% of patients include fractures (7% of patients; 6% of 290 adult patients and 10% of 154 pediatric patients).1
The safety of VITRAKVI was evaluated in 444 patients, in 3 clinical trials.1
aThe adverse reaction identifies a composite term.
bNational Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) v4.03.
cGrade 4 adverse reaction: 1 of cognitive impairment, 1 of pyrexia.
dIncludes: arthralgia, back pain, bone pain, flank pain, groin pain, growing pains, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal pain, musculoskeletal stiffness, myalgia, neck pain, non-cardiac chest pain, pain in extremity, pain in jaw, and tendon pain.
eIncludes: fatigue, asthenia.
fIncludes: face edema, generalized edema, lip edema, localized edema, edema, edema genital, edema peripheral, periorbital edema, and swelling.
gIncludes: cough, productive cough, and upper-airway cough syndrome.
hIncludes: dyspnea, and dyspnea exertional.
iIncludes: dizziness, dizziness postural, and vertigo.
jIncludes: amnesia, aphasia, cognitive disorder, confusional state, delirium, disturbance in attention, hallucination, hallucination visual, memory impairment, mental impairment, mental status changes.
kIncludes: abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, abdominal tenderness, epigastric discomfort, and gastrointestinal pain.
lIncludes: dermatitis, dermatitis acneiform, dermatitis bullous, dermatitis exfoliative generalized, eczema, eczema asteatotic, palmar-plantar erythrodysesthesia syndrome, rash, rash erythematous, rash macular, rash maculopapular, rash papular, rash pruritic, and rash pustular.
mIncludes: agitation, anxiety, depression, depressed mood, euphoric mood, fear, feeling jittery, irritability, panic attack, psychomotor hyperactivity, restlessness.
nIncludes: insomnia, sleep disorder, somnolence.
oIncludes: cystitis, cystitis escherichia, escherichia urinary tract infection, kidney infection, pyelonephritis, pyelonephritis acute, pyelonephritis chronic, and urinary tract infection.
pALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase.
Laboratory Abnormalities Occurring in ≥20% of Patients Treated With VITRAKVI1
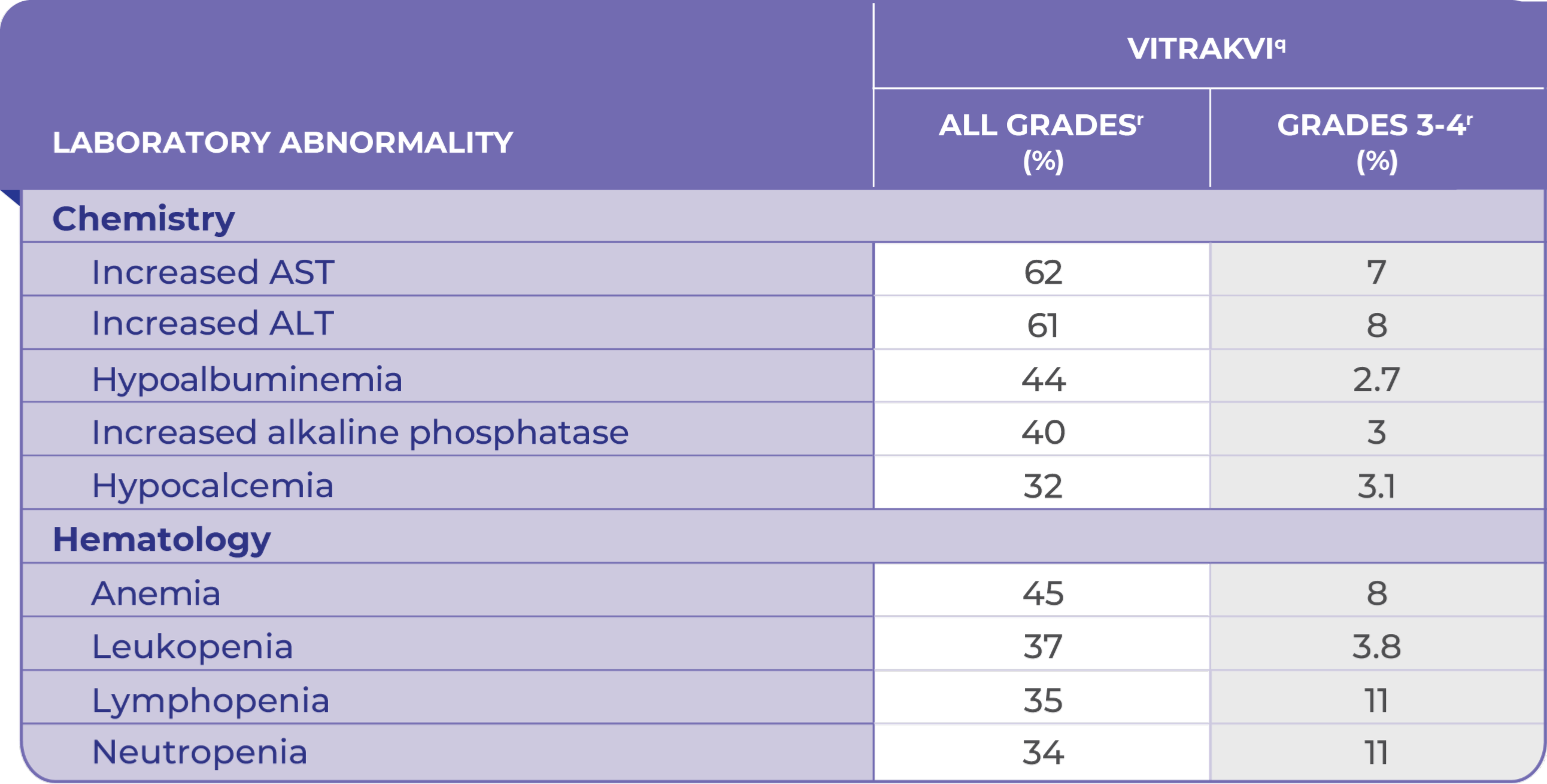
qBased on NCI-CTCAE v4.03.1
rDenominator for each laboratory parameter is based on the number of patients with a baseline and post treatment laboratory value available, which ranged from 416 to 442 patients.1
Reference
- VITRAKVI [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals, Inc.; April 2025. Return to content